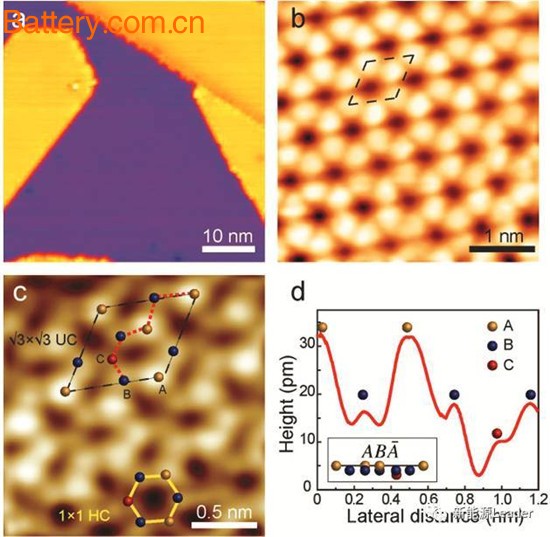
With the continuous development of lithium-ion battery technology, various new technologies and new materials emerge in an endless stream. Carbon nanotubes (CNT) and nano-carbon fiber (CNF) materials have been applied to lithium-ion batteries to improve the performance of lithium-ion batteries. Recently, graphene materials have made lithium-ion batteries the focus of public opinion. Although it turns out that these lithium-ion batteries with graphene names are just hype, they also make people pay attention to emerging materials on lithium-ion batteries. The possibility of application. Then we will bring you a new material - silene. The so-called silene is a nanosheet composed of a single layer of Si atoms and has a structure similar to that of graphene. Unlike graphene, the material can be directly used as a negative electrode material for lithium ion batteries, not just as an additive. The research results were jointly published by Jincheng Zhuang and others from Wollongong University in the latest issue of Advanced Material magazine. Due to its unique two-dimensional structure, silylene materials can provide sufficient space and channels for Li+ migration, thereby inhibiting the deformation of silylene materials during lithium insertion/delithiation. According to Jincheng Zhuang's research, the specific capacity of silene as a negative electrode material for lithium ion batteries can reach 954mAh/g. Although it is not as good as crystalline Si material, it is still far higher than traditional graphite materials (372mAh/g). The larger space, higher adsorption energy and lower Li+ diffusion barrier make silene the ideal anode material for high specific energy and long life lithium ion batteries. In the experiment, Jincheng Zhuang used molecular beam epitaxy (MBE) and solid phase reaction to synthesize silylene materials. We will not introduce the detailed synthesis process. Interested friends can ask for the original text. The following figure is an STM image of a silicon-based material synthesized by Jincheng Zhuang. From the figure, we can see that the honeycombed silicon-ene material exhibits a structure in which six Si atoms are not in the same plane. Above, two of the atoms are bent upwards, and one atom is bent downward, presenting an ABA-structure. At the same time, the massless carriers in the structure can greatly improve the conductivity of the silene material, thereby overcoming the problem that the conductivity of the Si anode material is not good for a long time. The use of Siene can greatly improve the silicon. The ability of the negative battery to charge and discharge with a large current. In the study of the stability of silylene materials, it is found that, unlike single-layer silene materials, multilayer silene materials are very stable in pure oxygen or air and are not easy to react with oxygen. The multi-layered silene material has a large specific surface area, which can significantly reduce the distance of Li diffusion. The larger space between the layers can also buffer the volume change caused by the lithium intercalation process, so it is an ideal anode material. The lithium intercalation properties of silylene materials have been simulated by software before synthesizing silene. The simulation results are shown in the figure below. Li atoms are suspended above Si atoms, and there are three closest Si atoms combined with them. As the amount of lithium increases, the Li atom insertion position is slightly shifted, and the rightmost Li0.5Si0.5 is regarded as a fully lithium-decomposed state of the silicon olefin material. Theoretical analysis shows that the theoretical specific capacity of silylene materials is about 954mAh/g, the volume expansion of fully embedded lithium is not more than 25%, and the diffusion energy barrier of Li+ in silene is very small. These characteristics make silylene materials a kind of Suitable lithium ion battery anode material. The synthesis method of multilayer silene is shown in the figure below. Jincheng Zhuang synthesized three kinds of multilayer silene materials by partial reaction method, which are completely silica oxide, partial silica oxide and non-silicene, among which non-silica. The material exhibits the best coulombic efficiency and cycle performance when used as a negative electrode material for lithium ion batteries. At the same time, the good conductivity of the silene material and the low Li+ diffusion energy barrier make the silylene material exhibit excellent rate performance. Since the silylene material prepared by the local reaction method currently contains a small amount of oxygen atoms, the cycle performance of the silene material can be improved by further increasing the purity of the silene material. Silicone materials are a new two-dimensional material that has emerged in recent years. Compared to traditional crystalline Si, silylene materials have many unique properties, such as good electrical conductivity and small volume expansion during lithium insertion. Siene materials have become a very promising anode material for lithium ion batteries. With the deepening of research on silicon-based materials, it will open up a blue ocean in the market of lithium-ion battery anode materials. Polycarboxylate Superplasticizer Traditional plasticizers are lignosulphonates as their sodium salt. Superplasticizers are synthetic polymers. Compounds used as superplasticizers include sulfonated naphthalene formaldehyde condensate, sulfonated melamine formaldehyde condensate, acetone formaldehyde condensate and polycarboxylate ethers. Polycarboxylate Superplasticizer,Polycarboxylic Superplasticizer,Pce Superplasticizer,Pce Liquid Polycarboxylate Superplasticizers Liaoning Kelong Fine Chemical Co.Ltd. , https://www.kelongchemy.com


Polycarboxylate Superplasticizer is used as a high range water reducing admixture in high performance concrete, high strength concrete, high volume fly ash/slag concrete, cement grouting, and dry mortar. They allow a water reduction up to 40% even with low dosage.
Silene will be the next generation silicon anode material?